Abstract
Hematopoietic stem cell transplant (HSCT) remains a curative option for many hematologic malignancies. However, disease relapse is still one of the major causes of death after transplant. Increasing the intensity of conditioning regimens may elicit more antitumor activity but not without additional risk of transplant-related morbidity and mortality. The use of total body irradiation (TBI), while it can be effective, carries the risk of long-term toxicity to extramedullary organs, as well as secondary cancers. We have previously demonstrated that the combination of myeloablative chemotherapy with intensity-modulated radiation therapy to the bone marrow, or TMI, delivered with a linear accelerator, does not increase the toxicity of transplant in the first year of follow up (PMID 29065747; PMID 25234438). However, the effects of TMI in long term survivors after hematopoietic stem cell transplant (HSCT) has not yet been described.
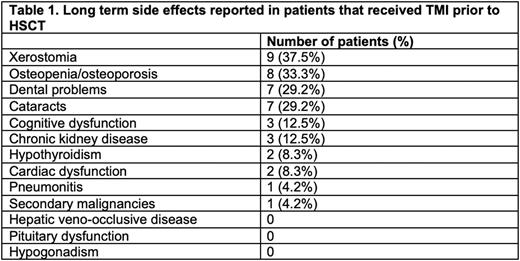
In this study, we retrospectively analyzed 24 consecutive patients who received HSCT with a TMI-based conditioning regimen between 2014 and 2021. The median age was 55.5 (range: 20-70) years. The median post-transplant follow up was 37.8 (range 12.0-89.7) months. Of 24 patients, 9 were diagnosed with multiple myeloma (MM) and received an autologous HSCT conditioned with high-dose Melphalan combined with either 3 (n=3), 6 (n=1) or 9 (n=5) Gy TMI. 15 (10 with acute myeloid leukemia, 3 with myelodysplastic syndrome and 2 with chronic myeloid leukemia) received an allogeneic HSCT from either a matched or 1-antigen mismatched related or unrelated donor, combining TMI 9 Gy with myeloablative fludarabine-IV busulfan. We then analyzed the incidence adverse events reported in published studies of TBI-based HSCT in our patient population (Table 1). In our long-term HSCT survivors receiving TMI-based conditioning, we observed xerostomia in 9 cases (37%, 3 of which had eating impairment), osteopenia or osteoporosis in 8 (33%, 2 of which also had fractures), dental in 29% and cataracts in 29%. Three subjects developed chronic kidney disease (12.5%, 2 patients developed stage 3 and 1 developed stage 4), 2 developed hypothyroidism (8%) and 2 patients developed heart failure (8%, both with mild decrease in ejection fraction without clinical decompensation). Only one patient (4%) developed a secondary malignancy (T-large granular lymphocytic leukemia). One patient (4%) developed a chronic interstitial pneumonitis, which was of unclear etiology and resolved spontaneously, but severe enough to require oxygen. These rates were overall lower than those historically reported with TBI (PMID 29482775) and can also be attributed to the standard chemotherapy utilized in the conditioning regimens.
In conclusion, TMI is a relatively safe treatment modality when included in myeloablative conditioning regimens for HSCT. The adverse events profile in our patient population was similar to that of TBI but with lower incidence rates for the majority of reported events. Phase 2 clinical trials using TMI in conditioning regimens are currently underway to assess HSCT outcomes and should guide its future use in HSCT.
Disclosures
Patel:Exelixis: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal